Pharmacovigilance

济民可信
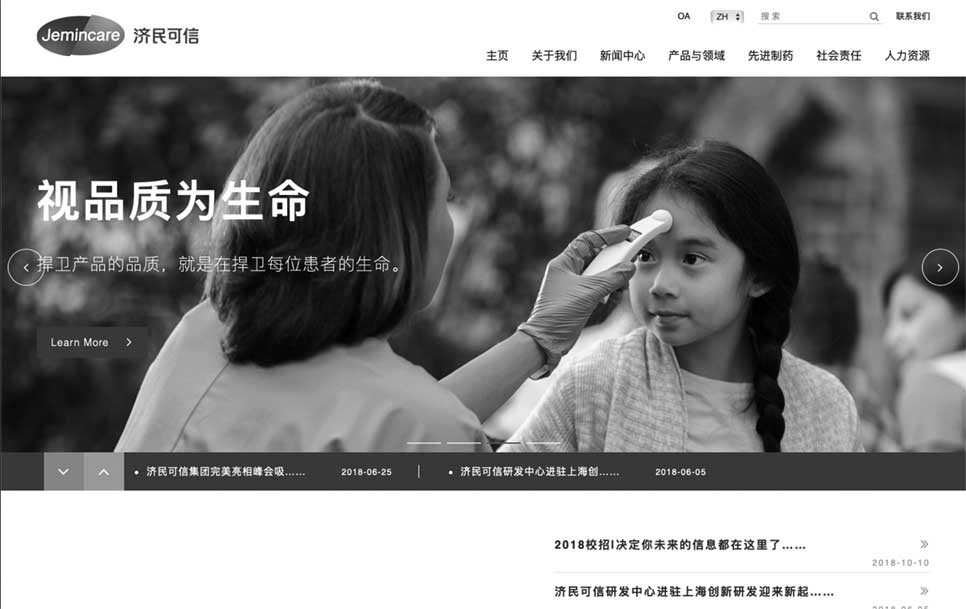
济民可信的存在价值,就是以济世之心,行惠民之举;诚信为品,造福天下。
江西济民可信集团有限公司创建于1999年,制药历史可追溯至上世纪五十年代,现有员工12000余名,总部位于中国南昌,在江西、北京、上海、江苏、浙江多地设有产业平台和研发机构。2017年,集团营业收入突破237亿元,跻身中国制药工业100强第6位、中国医药工业100强第10位。
秉承创新驱动的战略方针,公司专注于现代中药、化学药、生物制剂、保健品的研发、生产和销售,拥有7家制药基地、1座国际级博士后工作站、1个获CNAS认证的国家级实验室、5个省级工程技术中心,并在海外设立新药研发团队。当前,集团在心脑血管科、肾科、肿瘤科、抗感染及急救药物领域取得丰硕成果,核心产品“金水宝”、“醒脑静”、“康莱特”、“悉能”、“立幸”、“黄氏响声丸”、“九华痔疮栓”等在全国细分品类拥有较高市场份额。
Add to Favorites