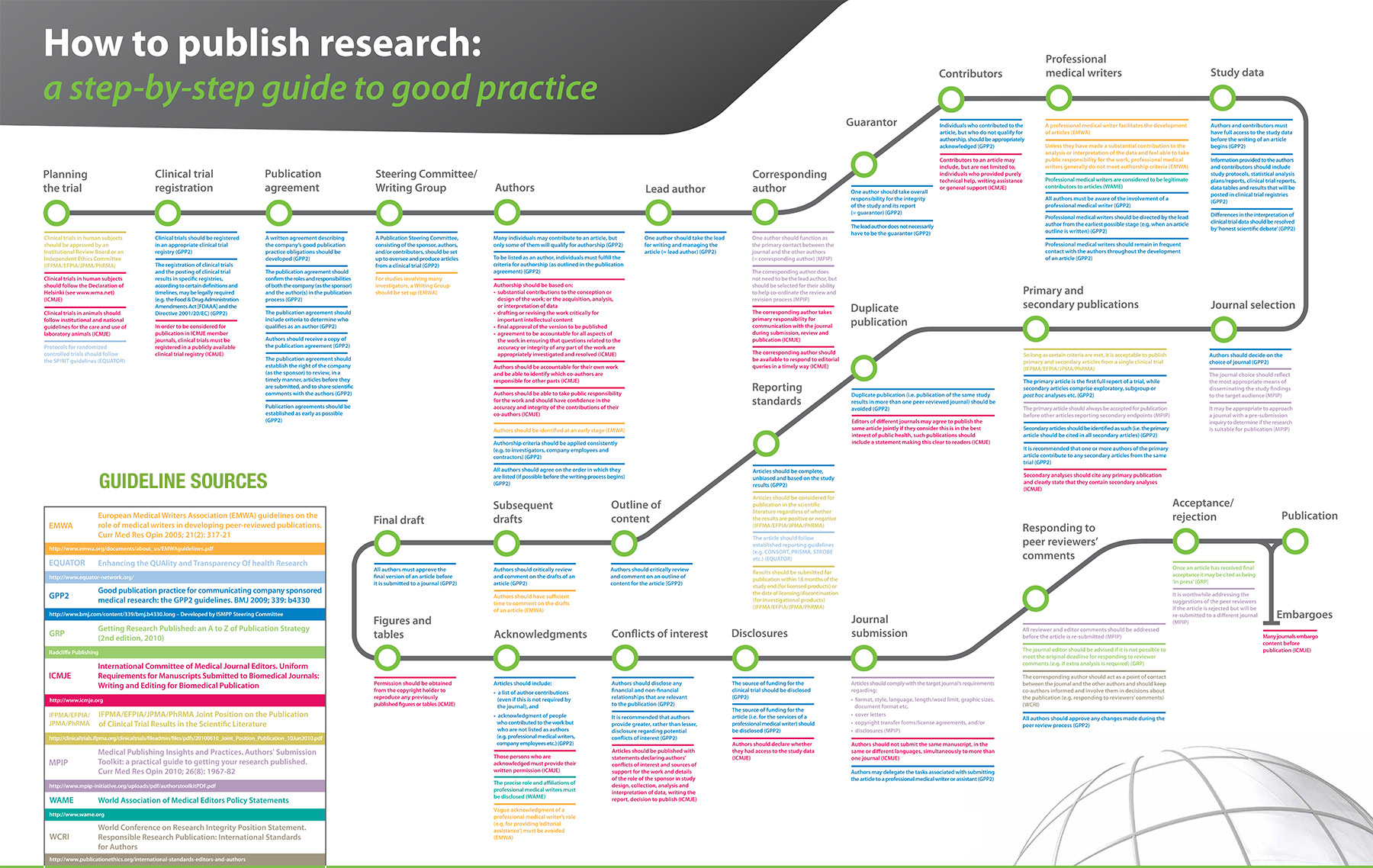
iProfiler™1
iPUB Station is now integrated with SYQUENCE to deliver the most comprehensive system for global medical communication teams. From Journal & Conference selection to Author Compliance and Document Publishing, SYQUENCE is guaranteed to streamline your publication development process and reduce resource workload. SYQUENCE ensures that your projects are completed on time with minimal effort and that published data is consistent.
-
simple
- Study data can be entered manually or the system can be integrated to automatically import data from CTMS, clinicaltrials or an excel data file. The system can be configured to instantly create default publication plans directly from the study metadata that is managed in iPUB Station
-
easy
- Simplicity drives our development teams to design tools that are intuitive for each user and that add value by making the processes and resource efforts easier. Automated task notifications are sent via email or instant messenger to alert users that they have a pending action.
-
fast
- The system includes an integrated training module that assists with user on-boarding. The migration utility instantly downloads sponsor records from clinicaltrials.gov and EudraCT. The unique system configuration enables users to modify records quickly and get them approved for publishing.
Digital Marketing
The HCA is run by its members for its members. We are proud of the work that we undertake on behalf of our membership and work hard to forge closer working relationships with other industry and NHS groups as well as the ABPI.
Focus on Digital Marketing
Our digital marketing team provides our customers with independent and overall services throughout the product life cycle.
What makes us different is our staff: high-quality medical team, production of high-quality medical professional content; experienced digital creative team, to provide customers with unique digital creativity.
Adapt to the changes of China's healthcare industry policies
China's pharmaceutical industry is facing rapid changes in industrial policies.
On the whole, the complianced, specialized and evidence-based pharmaceutical marketing model is the mainstream marketing model in the future.
We provide different digital marketing solutions for drugs / devices in different life cycles to ensure effectiveness and compliance:
- New Drugs:manage KOLs, obtain HEOR evidence and enter NRDL
- Chronic Drugs: patient management, extended DOT
- Special medicine and rare disease Drugs: expand to board market
Education & training
Develop comprehensive clinical training and education programs
MedSci has accumulated rich contents in medical education for many years, with more than 1000 independently developed courses and nearly 3000 courses in total
Clinical research: a series of in-depth clinical academic research courses, covering the whole process from topic selection, protocol design, fund application, data collection, statistical analysis, medical writing, submission and publication, 18 classic courses
Translational medicine: the latest and largest translational medicine conference in China, bringing the latest medical information from different perspectives of basic science, translational medicine and clinical application, 20 series of translational medicine conferences
Clinical practice: invite experts from all professional fields to bring clinical practice courses, gather their many years of clinical practice experience; at the same time, invite young doctors to bring unique perspectives
Medical Writing

High-Quality Medical Writing to Meet Your Medical Affairs and Communication Needs
Effective evidence and information dissemination is crucial for conveying product value to both internal and external stakeholders.
Our trained medical writers provide medical writing services for both pre-market and post-market stages of the product lifecycle, ensuring consistency in quality.
Our medical writers' documents are clear, concise, engaging, scientifically accurate, while ensuring full compliance with regulations, industry best practices, and corporate guidance.
Collaborative Approach to Meet Your Research Needs
Through collaboration with experts from various fields at Mace Medical, in all aspects of evidence generation, including health economics, patient-centered research, epidemiology, real-world evidence, interventional studies, market access, and broad therapeutic expertise,
Our medical writers gain deep insights into study design and implementation. Our unique positioning supports your overall research objectives through research documents and medical communications, including:
- Literature Search
- Study Protocols
- Publication Planning
- Abstract and Poster Writing
- Risk Management Plans
- Post-Market Research Reports

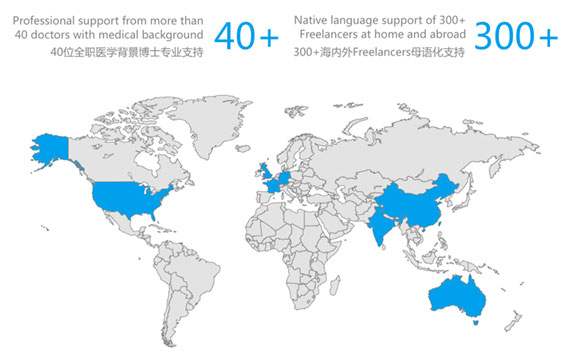
Our Medical Writing Experience
- 8,500+ SCI Paper Publication Support
- 300+ Study Protocols Written
- 1,000+ Slide Decks Written
- 40+ Full-time PhD Medical Background Support
- 300+ Global Freelance Native Language Support
- 10+ Years of Medical Writing Experience
Adherence to Clinical Research Guidelines
- CONSORT RCT Study Guidelines
- STROBE Epidemiological Observational Study Reporting Guidelines
- STARD Diagnostic Accuracy Reporting Standards
- PRISMA Systematic Review and META-Analysis Preferred Reporting
- TREND Non-Randomized Controlled Trial Reporting Guidelines
- ISPOR International Society for Pharmacoeconomics and Outcomes Research Ethics Guidelines
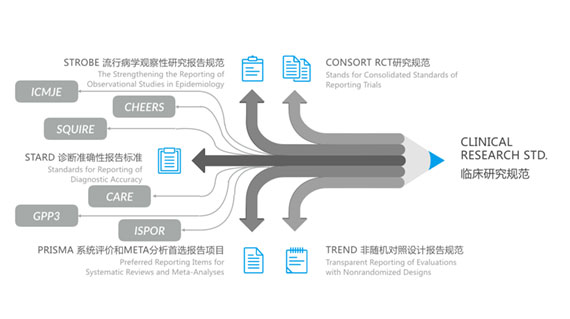
GPP3 - Good Publication Practice Guideline
Our work complies with the International Society for Medical Publication Professionals (ISMPP) Code of Ethics, and EMWA, EQUATOR, GRP, GPP3, ICMJE, IFPMA/EFPIA/JPMA/PhRMA, MPIP, WAME, and WCRI guidelines and policies will improve the quality of research which may form the basis for future health care.
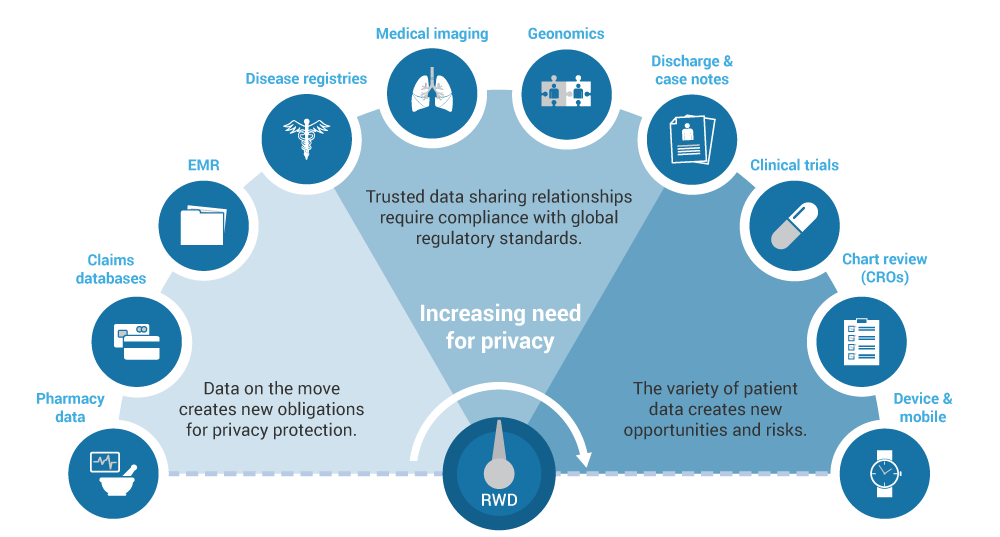
Real World Study
The Growing Demand for Real-World Evidence
Mees Medical's drug safety and pharmacovigilance team supports clinical trials and post-market safety monitoring for pharmaceutical and medical devices, providing independent and comprehensive services throughout the product lifecycle for our clients.
What sets us apart is our employees: highly qualified drug safety scientists and skilled medical professionals who have deep expertise in patient management, specific pharmacovigilance services, and regulatory affairs, with extensive experience in drug safety management.

We Can Assist You With:
-
Characterizing the natural history and progression of disease (e.g., incidence, prevalence, standards of care)
-
Identifying unmet clinical and human needs by describing the disease burden
-
Collecting data on rare disease populations
-
Quantifying real-world product-specific and/or comparative safety, efficacy, compliance, and other outcomes
-
Evaluating specific treatment patterns, quantifying related care costs, and populating health economics models
In-Depth Data Insights
We focus on your research questions and provide the best solutions that include a variety of real-world data sources and datasets. Our integrated team of health economists, epidemiologists, biostatisticians, and clinicians rigorously evaluates available databases to determine which ones provide the best information to meet your research needs. We have in-depth knowledge and experience with data sources from over 20 countries (including North America and Europe) as well as other regions such as Brazil, Japan, South Korea, China, Australia, and Taiwan. We are familiar with and can analyze large claims databases, electronic medical records, and combinations of these and other data sources.
We focus on your research questions and provide the best solutions that include a variety of real-world data sources and datasets. Our integrated team of health economists, epidemiologists, biostatisticians, and clinicians rigorously evaluates available databases to determine which ones provide the best information to meet your research needs. We have in-depth knowledge and experience with data sources from over 20 countries (including North America and Europe) as well as other regions such as Brazil, Japan, South Korea, China, Australia, and Taiwan. We are familiar with and can analyze large claims databases, electronic medical records, and combinations of these and other data sources.
In-Depth Collaboration with Professional Institutions, Associations, Societies, and KOLs
Achieving optimal market access and effective commercialization requires customized research design, in-depth expertise, and a network of relationships with national KOLs. Our understanding of the post-market environment, combined with over a decade of clinical research experience, translates into unique capabilities to provide tailored and effective research designs that meet specific goals, market demands, and product regulatory requirements. Additionally, our operating model is customized based on expert opinions from medical, clinical, project management, regulatory, healthcare, and epidemiology functions to meet the project-specific goals and stakeholder expectations.
Excellence in Research Design and Execution
- Extensive experience - Focused on real-world research design, operations, monitoring, and outcomes teams, 15 key clinical research areas, 200+ collaborative research sites, 500+ long-term collaborating researchers, 70,000+ chief physician members, and 2 million clinical physician resources
- Professional research team - Achieving significantly better research initiation and patient recruitment times compared to industry benchmarks when working under early-stage collaboration models
- Therapeutic area experts - Ability to handle a wide range of indications and leverage our network of clinicians and national operations professionals
- Through technology and collaborative innovation - Fully leveraging artificial intelligence and big data technology, as well as the characteristics of real-world research, significantly reducing research costs, improving operational efficiency, and ensuring high-quality research outcomes
Post Market Study
Professional team helps post-marketing study in China
Our real-world research BU has extensive experience in the design and implementation of PMS. Studies produce influential medical data through structured research design and implementation, as well as evidence of products or specific patient groups, to better guide clinical practice, achieve the most market access and optimal business, and meet the requirements of regulatory authorities. All studies were conducted in accordance with the GCP specifications and the corresponding research guidelines.
We develop streamlined and cost-effective standard operating procedures for PMS to maximize data collection, monitoring, and quality, while minimizing operational risk.
Our real-world research team has rich knowledge of policy and expertise, including ethical and regulatory requirements, patient privacy legislation, investigator assistance policies, academic and website networks, patient associations, national health databases, and local healthcare systems.
Excellence in design and operation
Mees Medical's real-world research BU has extensive experience in the design and execution of post-marketing clinical research. Our research generates influential medical data through structured research design and execution, as well as medical evidence for products or specific patient groups, to better guide clinical practice, achieve the most market access and optimal business, and meet the requirements of regulatory authorities. All of our studies are conducted according to GCP specifications and corresponding research guidelines.
Mees Medical develops streamlined and cost-effective standard operating procedures for post-marketing studies, maximizing data collection, monitoring, and quality, while minimizing operational risks.
Mees Medical's real-world research team possesses deep policy and professional knowledge, including ethical and regulatory requirements, patient privacy legislation, investigator subsidy policies, academic and website networks, patient associations, national health databases, and local healthcare systems.
Key Drug Monitoring
Mees Medical's real-world research BU has extensive experience in the design and execution of post-marketing clinical research. Our research generates influential medical data through structured research design and execution, as well as medical evidence for products or specific patient groups, to better guide clinical practice, achieve the most market access and optimal business, and meet the requirements of regulatory authorities. All of our studies are conducted according to GCP specifications and corresponding research guidelines.
Mees Medical develops streamlined and cost-effective standard operating procedures for post-marketing studies, maximizing data collection, monitoring, and quality, while minimizing operational risks.
Mees Medical's real-world research team possesses deep policy and professional knowledge, including ethical and regulatory requirements, patient privacy legislation, investigator subsidy policies, academic and website networks, patient associations, national health databases, and local healthcare systems.