Medical Writing

High-Quality Medical Writing to Meet Your Medical Affairs and Communication Needs
Effective evidence and information dissemination is crucial for conveying product value to both internal and external stakeholders.
Our trained medical writers provide medical writing services for both pre-market and post-market stages of the product lifecycle, ensuring consistency in quality.
Our medical writers' documents are clear, concise, engaging, scientifically accurate, while ensuring full compliance with regulations, industry best practices, and corporate guidance.
Collaborative Approach to Meet Your Research Needs
Through collaboration with experts from various fields at Mace Medical, in all aspects of evidence generation, including health economics, patient-centered research, epidemiology, real-world evidence, interventional studies, market access, and broad therapeutic expertise,
Our medical writers gain deep insights into study design and implementation. Our unique positioning supports your overall research objectives through research documents and medical communications, including:
- Literature Search
- Study Protocols
- Publication Planning
- Abstract and Poster Writing
- Risk Management Plans
- Post-Market Research Reports

Our Medical Writing Experience
- 8,500+ SCI Paper Publication Support
- 300+ Study Protocols Written
- 1,000+ Slide Decks Written
- 40+ Full-time PhD Medical Background Support
- 300+ Global Freelance Native Language Support
- 10+ Years of Medical Writing Experience
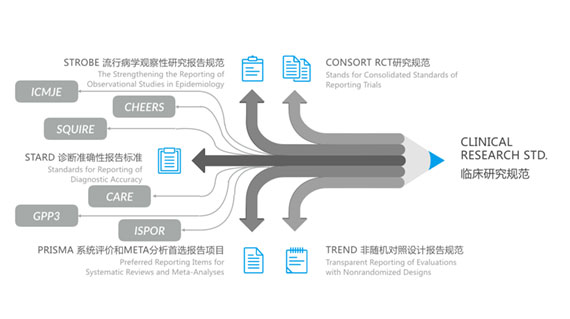
Adherence to Clinical Research Guidelines
- CONSORT RCT Study Guidelines
- STROBE Epidemiological Observational Study Reporting Guidelines
- STARD Diagnostic Accuracy Reporting Standards
- PRISMA Systematic Review and META-Analysis Preferred Reporting
- TREND Non-Randomized Controlled Trial Reporting Guidelines
- ISPOR International Society for Pharmacoeconomics and Outcomes Research Ethics Guidelines