Medical Writing

High-Quality Medical Writing to Meet Your Medical Affairs and Communication Needs
Effective evidence and information dissemination is crucial for conveying product value to both internal and external stakeholders.
Our trained medical writers provide medical writing services for both pre-market and post-market stages of the product lifecycle, ensuring consistency in quality.
Our medical writers' documents are clear, concise, engaging, scientifically accurate, while ensuring full compliance with regulations, industry best practices, and corporate guidance.
Collaborative Approach to Meet Your Research Needs
Through collaboration with experts from various fields at Mace Medical, in all aspects of evidence generation, including health economics, patient-centered research, epidemiology, real-world evidence, interventional studies, market access, and broad therapeutic expertise,
Our medical writers gain deep insights into study design and implementation. Our unique positioning supports your overall research objectives through research documents and medical communications, including:
- Literature Search
- Study Protocols
- Publication Planning
- Abstract and Poster Writing
- Risk Management Plans
- Post-Market Research Reports

Our Medical Writing Experience
- 8,500+ SCI Paper Publication Support
- 300+ Study Protocols Written
- 1,000+ Slide Decks Written
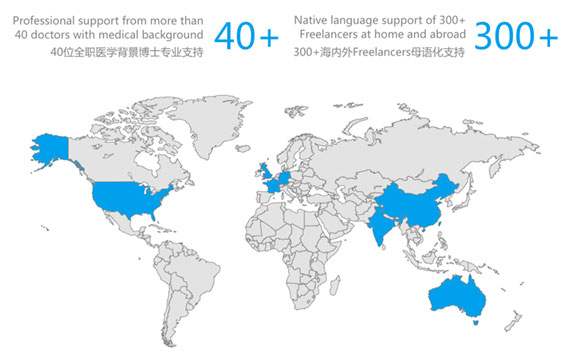
- 40+ Full-time PhD Medical Background Support
- 300+ Global Freelance Native Language Support
- 10+ Years of Medical Writing Experience
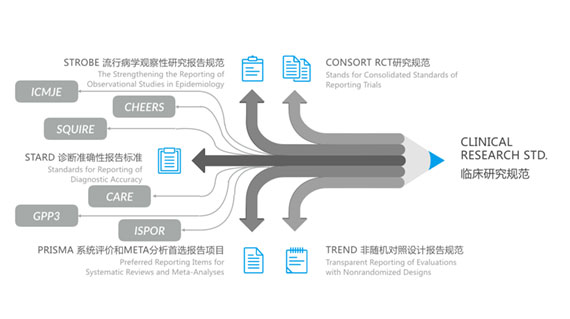
Adherence to Clinical Research Guidelines
- CONSORT RCT Study Guidelines
- STROBE Epidemiological Observational Study Reporting Guidelines
- STARD Diagnostic Accuracy Reporting Standards
- PRISMA Systematic Review and META-Analysis Preferred Reporting
- TREND Non-Randomized Controlled Trial Reporting Guidelines
- ISPOR International Society for Pharmacoeconomics and Outcomes Research Ethics Guidelines
Education & training
Develop comprehensive clinical training and education programs
MedSci has accumulated rich contents in medical education for many years, with more than 1000 independently developed courses and nearly 3000 courses in total
Clinical research: a series of in-depth clinical academic research courses, covering the whole process from topic selection, protocol design, fund application, data collection, statistical analysis, medical writing, submission and publication, 18 classic courses
Translational medicine: the latest and largest translational medicine conference in China, bringing the latest medical information from different perspectives of basic science, translational medicine and clinical application, 20 series of translational medicine conferences
Clinical practice: invite experts from all professional fields to bring clinical practice courses, gather their many years of clinical practice experience; at the same time, invite young doctors to bring unique perspectives
GPP3 - Good Publication Practice Guideline
Our work complies with the International Society for Medical Publication Professionals (ISMPP) Code of Ethics, and EMWA, EQUATOR, GRP, GPP3, ICMJE, IFPMA/EFPIA/JPMA/PhRMA, MPIP, WAME, and WCRI guidelines and policies will improve the quality of research which may form the basis for future health care.