iPUB Station is now integrated with SYQUENCE to deliver the most comprehensive system for global medical communication teams. From Journal & Conference selection to Author Compliance and Document Publishing, SYQUENCE is guaranteed to streamline your publication development process and reduce resource workload. SYQUENCE ensures that your projects are completed on time with minimal effort and that published data is consistent.
-
simple
- Study data can be entered manually or the system can be integrated to automatically import data from CTMS, clinicaltrials or an excel data file. The system can be configured to instantly create default publication plans directly from the study metadata that is managed in iPUB Station
-
easy
- Simplicity drives our development teams to design tools that are intuitive for each user and that add value by making the processes and resource efforts easier. Automated task notifications are sent via email or instant messenger to alert users that they have a pending action.
-
fast
- The system includes an integrated training module that assists with user on-boarding. The migration utility instantly downloads sponsor records from clinicaltrials.gov and EudraCT. The unique system configuration enables users to modify records quickly and get them approved for publishing.
The HCA is run by its members for its members. We are proud of the work that we undertake on behalf of our membership and work hard to forge closer working relationships with other industry and NHS groups as well as the ABPI.
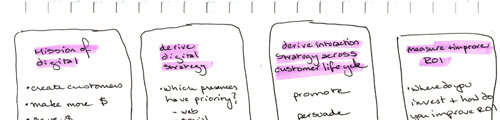
Focus on Digital Marketing
Our digital marketing team provides our customers with independent and overall services throughout the product life cycle.
What makes us different is our staff: high-quality medical team, production of high-quality medical professional content; experienced digital creative team, to provide customers with unique digital creativity.
Adapt to the changes of China's healthcare industry policies
China's pharmaceutical industry is facing rapid changes in industrial policies.
On the whole, the complianced, specialized and evidence-based pharmaceutical marketing model is the mainstream marketing model in the future.
We provide different digital marketing solutions for drugs / devices in different life cycles to ensure effectiveness and compliance:
- New Drugs:manage KOLs, obtain HEOR evidence and enter NRDL
- Chronic Drugs: patient management, extended DOT
- Special medicine and rare disease Drugs: expand to board market