Market Access
Helping You Formulate a Market Access Strategy that Maximizes Access for Your Brand
With more expensive drugs winning regulatory approval and increased scrutiny on drug pricing, market access has never been more challenging and competitive. Our team offers a full spectrum of solutions and services to help you navigate the market access landscape and maximize access for your brand.
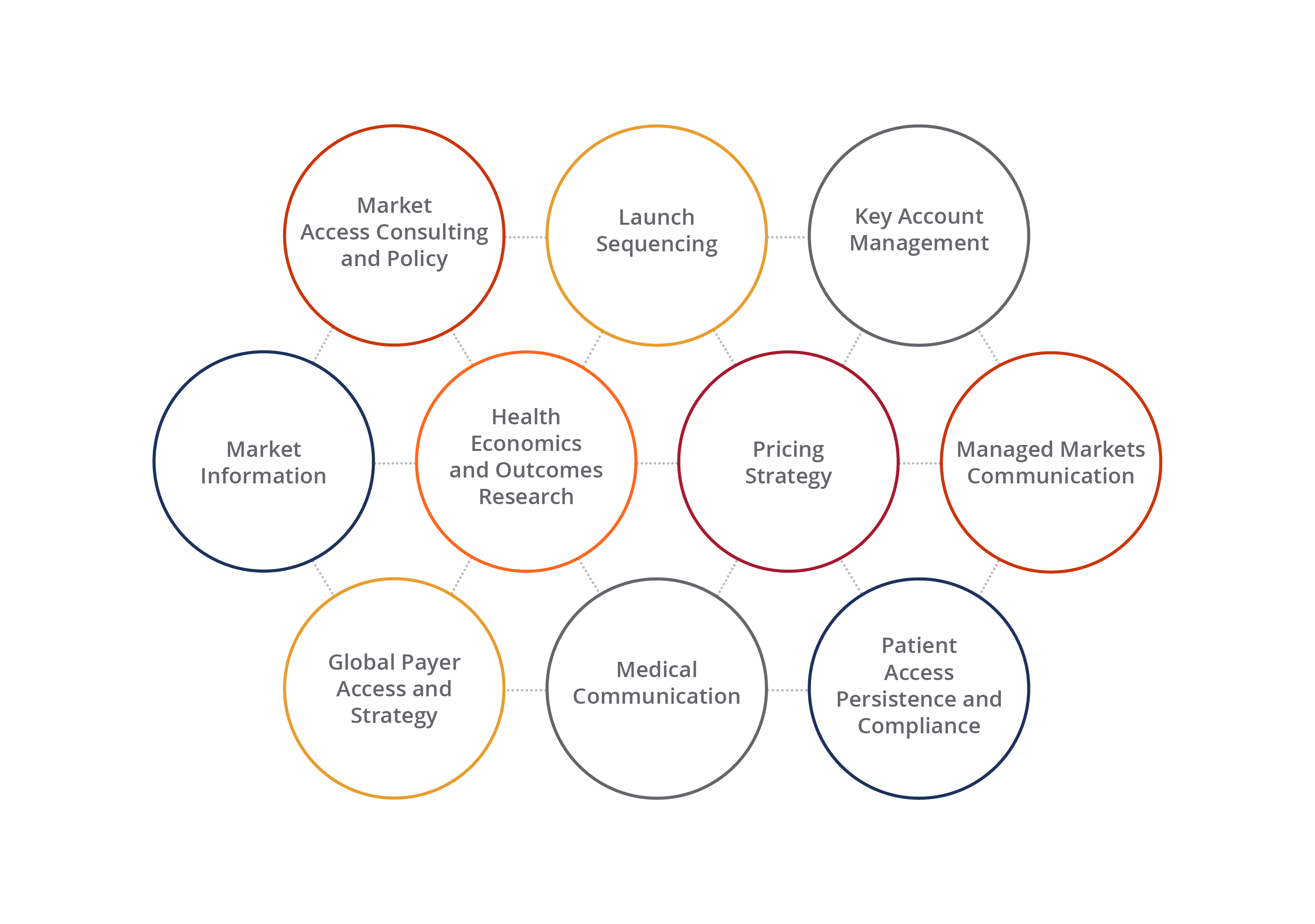
Optimizing Access to Markets Throughout Your Brand’s Life Cycle
Wherever you are in your brand’s life cycle, there is a team that can help you to achieve your access-related objectives. From research to strategy and tactical execution, we offer a comprehensive suite of services to give your brand and organization a competitive edge every step of the way.
A Complete Toolbox for Global Market Access
Whether it’s gathering market information or conducting cost analyses, we have the depth and resources to give our customers the insights they want and need. We bring these insights to life through our analytics, as well as our creative executions, generating captivating content that best communicates our customers’ strategy-driven messages.
Our Global Market Access Offering:
- Market assessment and analysis
- Comparative effectiveness research
- Pricing reimbursement and HEOR
- HCEI communications
- Patient assistance services
- Legislative and regulatory analysis
A Fit for All Shapes and Sizes
Whether you’re working for a company with a history of blockbuster launches or a start-up with a promising pipeline, we can help you develop and implement your market access strategy. Our multiple teams have collective experience across the healthcare continuum and can provide the support and services specific to your organization and brand, always tailoring them to help you overcome your brand-specific access challenges.
Close Connections on a Global Scale
With experienced teams in multiple countries, we understand the policies, trends and nuances in markets around the world.
BUILDING AND COMMUNICATING STRONG AND EFFECTIVE VALUE PROPOSITIONS
The complex, heterogeneous, and rapidly-changing payer environment is becoming increasingly difficult to navigate. Our expert teams of industry leaders, former payers, policy experts, and experienced strategists can help you navigate complex evidence requirements and anticipate and overcome payer barriers to help you achieve optimal price and access. Our team of communication specialists can help you develop compelling arguments presented in an accurate, succinct, and engaging story format to shape effective payer positioning and position evidence to overcome payer objections.
WE CAN HELP YOU
Gather in-depth information on the HTA, pricing, and reimbursement drivers and limiters, as well as landscape reviews, analog and market assessments
- Develop comprehensive global pricing and market access strategies and recommendations
- Develop evidence generation strategies to support optimal value positioning, HTA assessment, and pricing
- Support in-licensing decisions with rapid turnaround insights into global pricing and reimbursement potential
- Provide cross-functional alignment on pricing, access and evidence strategies through market access workshops
- Craft value stories that articulate a logical flow of arguments, supported by available evidence
- Test, refine, and validate value messages with relevant stakeholders
- Present payer-relevant evidence through global value dossiers, slide decks, objection handlers, and training
- Develop country-specific pricing and reimbursement or formulary submissions, including Academy of Managed Care Pharmacy (AMCP) dossiers for U.S. payers and HTA submissions for various markets
- Convey messages and evidence in a dynamic, user-friendly way electronically through iValue Suite
- Explore key market access hypotheses through international payer advisory boards
WE OFFER YOU
- A network of over 2,000 payer experts and advisors worldwide
- A Pricing and Reimbursement Policy Council (PRPC) comprised of a diverse group of industry experts, and providing insights into the latest issues, trends, and changes affecting market access
- Expertise and experience in over 45 countries and across all major therapeutic areas
- In-house expertise and no outsourcing, with ~35,000 payer interviews and all value story and dossier writing done internally
- Rigorous quality standards applied to all content development
ADDRESSING THE LATEST TRENDS IN MARKET ACCESS
- Advanced Therapy Medicinal Products
- Early Access
- Expanded Access
- Conditional Marketing (e.g,, for Orphan Drugs)
- Medical Devices
- Price Referencing
- Surrogate Endpoint Use
- Transparency in Drug Pricing
Operation Management
Effective communication of evidence and information is essential to conveying the value of your products, both to internal and external stakeholders. With approximately 20 years of experience, our highly trained staff provide medical writing services for the peri- and post-approval phases of the product lifecycle, guaranteeing a consistency in quality and voice. Our writers prepare documents that are clear, concise, compelling, and scientifically accurate, while also ensuring they are fully compliant with regulations, industry best practices, and your corporate guidance.
- Site Identification
- KOLs networking
- PI Training
- Clinical Audit
- online SMO
Real World Data
Effective communication of evidence and information is essential to conveying the value of your products, both to internal and external stakeholders. With approximately 20 years of experience, our highly trained staff provide medical writing services for the peri- and post-approval phases of the product lifecycle, guaranteeing a consistency in quality and voice. Our writers prepare documents that are clear, concise, compelling, and scientifically accurate, while also ensuring they are fully compliant with regulations, industry best practices, and your corporate guidance.
- Real-world data collection
- patient or physician surveys
- Hybrid database studies
- Product Registries
- Disease Registries
- Electronic clinical outcome assessment eCOA (ePRO/eClniRO/eObsRO)
Pharmacovigilance
Our work complies with the International Society for Medical Publication Professionals (ISMPP) Code of Ethics, and EMWA, EQUATOR, GRP, GPP3, ICMJE, IFPMA/EFPIA/JPMA/PhRMA, MPIP, WAME, and WCRI guidelines and policies will improve the quality of research which may form the basis for future health care.
Post Market Study
Professional team helps post-marketing study in China
Our real-world research BU has extensive experience in the design and implementation of PMS. Studies produce influential medical data through structured research design and implementation, as well as evidence of products or specific patient groups, to better guide clinical practice, achieve the most market access and optimal business, and meet the requirements of regulatory authorities. All studies were conducted in accordance with the GCP specifications and the corresponding research guidelines.
We develop streamlined and cost-effective standard operating procedures for PMS to maximize data collection, monitoring, and quality, while minimizing operational risk.
Our real-world research team has rich knowledge of policy and expertise, including ethical and regulatory requirements, patient privacy legislation, investigator assistance policies, academic and website networks, patient associations, national health databases, and local healthcare systems.
Excellence in design and operation
Mees Medical's real-world research BU has extensive experience in the design and execution of post-marketing clinical research. Our research generates influential medical data through structured research design and execution, as well as medical evidence for products or specific patient groups, to better guide clinical practice, achieve the most market access and optimal business, and meet the requirements of regulatory authorities. All of our studies are conducted according to GCP specifications and corresponding research guidelines.
Mees Medical develops streamlined and cost-effective standard operating procedures for post-marketing studies, maximizing data collection, monitoring, and quality, while minimizing operational risks.
Mees Medical's real-world research team possesses deep policy and professional knowledge, including ethical and regulatory requirements, patient privacy legislation, investigator subsidy policies, academic and website networks, patient associations, national health databases, and local healthcare systems.
Key Drug Monitoring
Mees Medical's real-world research BU has extensive experience in the design and execution of post-marketing clinical research. Our research generates influential medical data through structured research design and execution, as well as medical evidence for products or specific patient groups, to better guide clinical practice, achieve the most market access and optimal business, and meet the requirements of regulatory authorities. All of our studies are conducted according to GCP specifications and corresponding research guidelines.
Mees Medical develops streamlined and cost-effective standard operating procedures for post-marketing studies, maximizing data collection, monitoring, and quality, while minimizing operational risks.
Mees Medical's real-world research team possesses deep policy and professional knowledge, including ethical and regulatory requirements, patient privacy legislation, investigator subsidy policies, academic and website networks, patient associations, national health databases, and local healthcare systems.











