Real World Study
The Growing Demand for Real-World Evidence
Mees Medical's drug safety and pharmacovigilance team supports clinical trials and post-market safety monitoring for pharmaceutical and medical devices, providing independent and comprehensive services throughout the product lifecycle for our clients.
What sets us apart is our employees: highly qualified drug safety scientists and skilled medical professionals who have deep expertise in patient management, specific pharmacovigilance services, and regulatory affairs, with extensive experience in drug safety management.

We Can Assist You With:
-
Characterizing the natural history and progression of disease (e.g., incidence, prevalence, standards of care)
-
Identifying unmet clinical and human needs by describing the disease burden
-
Collecting data on rare disease populations
-
Quantifying real-world product-specific and/or comparative safety, efficacy, compliance, and other outcomes
-
Evaluating specific treatment patterns, quantifying related care costs, and populating health economics models
In-Depth Data Insights
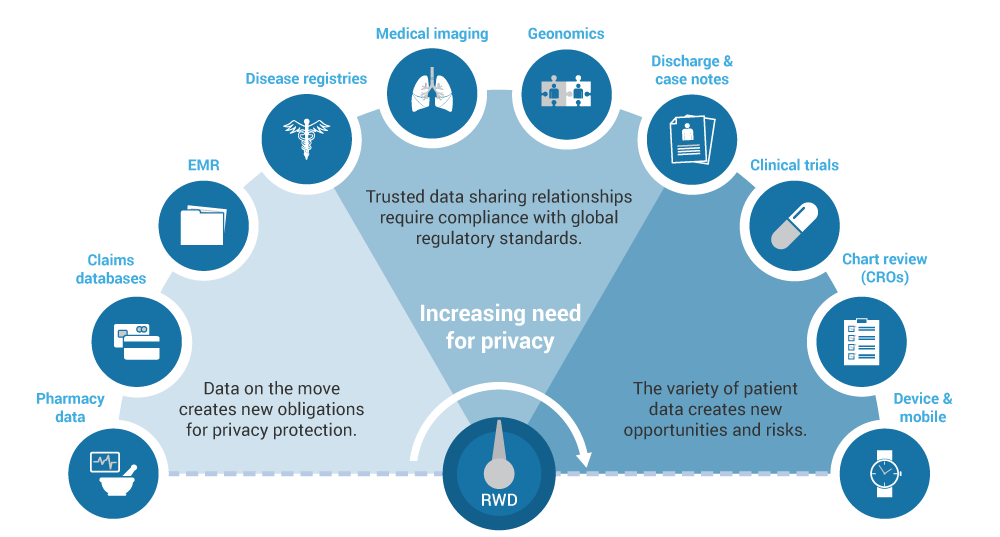
We focus on your research questions and provide the best solutions that include a variety of real-world data sources and datasets. Our integrated team of health economists, epidemiologists, biostatisticians, and clinicians rigorously evaluates available databases to determine which ones provide the best information to meet your research needs. We have in-depth knowledge and experience with data sources from over 20 countries (including North America and Europe) as well as other regions such as Brazil, Japan, South Korea, China, Australia, and Taiwan. We are familiar with and can analyze large claims databases, electronic medical records, and combinations of these and other data sources.
We focus on your research questions and provide the best solutions that include a variety of real-world data sources and datasets. Our integrated team of health economists, epidemiologists, biostatisticians, and clinicians rigorously evaluates available databases to determine which ones provide the best information to meet your research needs. We have in-depth knowledge and experience with data sources from over 20 countries (including North America and Europe) as well as other regions such as Brazil, Japan, South Korea, China, Australia, and Taiwan. We are familiar with and can analyze large claims databases, electronic medical records, and combinations of these and other data sources.
In-Depth Collaboration with Professional Institutions, Associations, Societies, and KOLs
Achieving optimal market access and effective commercialization requires customized research design, in-depth expertise, and a network of relationships with national KOLs. Our understanding of the post-market environment, combined with over a decade of clinical research experience, translates into unique capabilities to provide tailored and effective research designs that meet specific goals, market demands, and product regulatory requirements. Additionally, our operating model is customized based on expert opinions from medical, clinical, project management, regulatory, healthcare, and epidemiology functions to meet the project-specific goals and stakeholder expectations.
Excellence in Research Design and Execution
- Extensive experience - Focused on real-world research design, operations, monitoring, and outcomes teams, 15 key clinical research areas, 200+ collaborative research sites, 500+ long-term collaborating researchers, 70,000+ chief physician members, and 2 million clinical physician resources
- Professional research team - Achieving significantly better research initiation and patient recruitment times compared to industry benchmarks when working under early-stage collaboration models
- Therapeutic area experts - Ability to handle a wide range of indications and leverage our network of clinicians and national operations professionals
- Through technology and collaborative innovation - Fully leveraging artificial intelligence and big data technology, as well as the characteristics of real-world research, significantly reducing research costs, improving operational efficiency, and ensuring high-quality research outcomes
药物警戒
聚焦药物安全和药物警戒
梅斯医学的药物安全和药物警戒团队支持药械的临床试验和上市后安全监查,为我们客户提供产品整个生命周期内独立和整体服务。
让我们与众不同的是我们的员工:高素质的药物安全科学家和熟练的医疗专业人员,他们在患者管理,特定的药物警戒服务以及监管法规方面,拥有深厚的专业知识和药物安全管理丰富的经验。
如何应对中国药物安全和药物警戒的挑战?
我们建立长期,信任的关系,并根据您的需求提供个性化的服务。没有两个客户是相同的,我们的理念是塑造我们的支持,以最好地满足您的需求和结构。全面而言,我们专注于为合规和准确的全球批准前和批准后药物警戒提供有效的解决方案。
无论您是在寻求全面的还是独立的药物警戒服务,我们都可以在药物安全和药物警戒方面实施创新解决方案。
我们在产品/器械开发的任何阶段管理您的药物警戒需求,以确保质量和合规性:
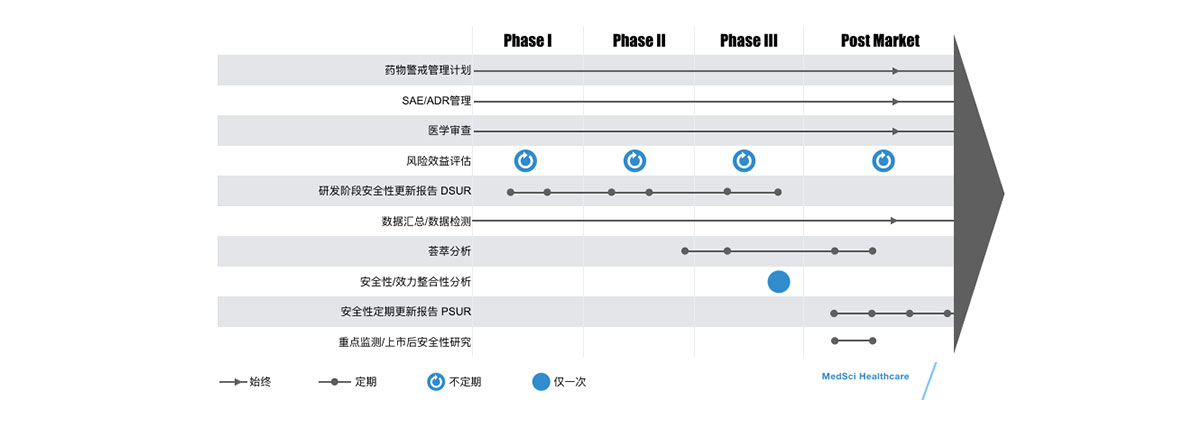
- 临床试验安全性:第I-IV期,从数据录入到个例结束,全面处理,包括加急/定期报告, CED直报
- 上市后安全性:AE个例处理,报告,信号检测,呼叫中心,文献检索和审查,产品投诉, 医学翻译
- 药物警戒医师:我们在安全和药物警戒的生命周期的各个方面都有专门/训练有素的医生
- 药物警戒数据库系统:最新的iDrugSafety药物警戒数据库系统,以及专业的支持团队,具有在客户和内部安全数据库中工作的专业知识
我们拥有业内最专业的药物警戒团队之一,在全国范围内为您服务
每个项目都由一个药物警戒专业人员团队提供支持,这些专业人员战略性地支持特定需求。当地员工根据需要支持监管报告,以确保合规性。无论位于何处,我们都遵循全球流程以确保整个团队的一致性。 我们的员工拥有强大的学术背景,包括药剂师,医生,护士和生命科学毕业生。在我们多年的药物警戒运营期间,我们的团队已经深入了解立法和广泛的行业经验。
在不断变化的监管环境中,我们在准时安全报告中实现了> 99%的合规率。
近年来,药物警戒立法变得比以往任何时候都更加严格。报告需要更加强大和专业化,因此客户越来越多地将安全和药物警戒服务外包出去。我们可以提供:
*具有预编程监管报告要求的自动化安全报告分发系统。向监管机构,道德委员会和调查站点提供适当,安全和可控的快速和定期报告,并提供全面的跟踪和审计跟踪
*符合ICH E2B标准的药物警戒数据库系统,可生成标准监管报告,并可灵活定制以满足您的特定要求
*专门的监管安全情报小组可以访问监管情报数据库,可以持续监控全球的监管要求
"为了应对国内药物相关政策的快速变化,我们与梅斯医学在药物警戒方面进行合作,他们高效的安全性数据库和专业药物警戒服务让我们快速的建立起合规的药物警戒体系"











